Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
Drug Discovery
Covid-19
To conquer COVID-19, create the perfect pill
Experts say oral antivirals, long out of reach, could play a critical role in extinguishing the current coronavirus pandemic and preventing others from emerging
by Bethany Halford
May 20, 2021
| A version of this story appeared in
Volume 99, Issue 19

Credit: Luisa Jung
As the COVID-19 crisis unfurled, scientists accomplished feats once considered impossible. Cheap, reliable COVID-19 tests are now readily available in many parts of the world. And the fact that hundreds of millions of people have taken safe and effective vaccines for a virus first identified only 18 months ago is a marvel of modern medicine.
In brief
As COVID-19 continues to take a global toll, drugmakers are racing to develop antiviral pills that could turn the tide of the pandemic. But the road to developing an antiviral treatment is long and hard. Often, promising candidates never make it to patients. Read on to learn about the most advanced SARS-CoV-2 antiviral candidates and what makes drugging viruses such a challenge.
But those advances may not be enough to end the pandemic.
COVID-19 isn’t going away, says Sarah Read, the deputy director of the Division of AIDS at the US National Institute of Allergy and Infectious Diseases (NIAID). Many countries don’t have access to enough doses of vaccine to come close to immunizing the masses. Low- and lower-middle-income countries, which account for almost half the world’s population, have received only 17% of COVID-19 vaccines, according to the World Health Organization. Even places where vaccination rates are high—Israel, the UK, and the US—continue to see infections, albeit at a low level. What’s more, some proportion of the population, which Read says could be as high as 20% in the US, either can’t or will choose not to be vaccinated.
COVID-19 could linger for years as many remain unvaccinated and as the virus continues to mutate, potentially making variants that render vaccines less effective. Doctors and public health experts say they’re still missing one key tool—an oral antiviral.
“There’s always going to be a need for another mechanism of protection,” says Read, who for the past year has focused on evaluating clinical therapeutics for COVID-19. “What we need to really make a dent in the pandemic is to be able to treat people very early in the course of their illness, right when they’re diagnosed: give them a safe, oral antiviral they can start immediately and prevent them winding up in the hospital, prevent them from dying, and prevent those strains on the health-care system.”
Making antivirals specifically for infections of SARS-CoV-2—the virus that causes COVID-19—or for acute viral infections in general has been tough. Antivirals are effective for only a short period between infection and full-blown disease. What’s more, it usually takes years to develop an antiviral and bring it to market. Often, public health measures eradicate a virus before a new antiviral can become available.
Approved antivirals that predate the current pandemic either haven’t been effective at stopping SARS-CoV-2 infections or have had only limited success. Nevertheless, drugmakers continue to pursue the elusive goal of a pill that kills SARS-CoV-2. Gilead Sciences is hoping to tweak its antiviral drug remdesivir (marketed as Veklury) so that it can be given orally. Merck & Co. is pouring resources into molnupiravir, a compound that was in early development at the Emory Institute for Drug Development for other diseases before the COVID-19 pandemic. And Pfizer started developing its oral SARS-CoV-2 inhibitor PF-07321332 from scratch.
Being able to give a pill to people experiencing the first symptoms of a SARS-CoV-2 infection or to people who know they’ve been exposed to the virus could give doctors a way to intervene early in the course of the disease, says physician Mangala Narasimhan, director of critical care services at Northwell Health in New York.
“We find antivirals for the flu, like Tamiflu, to be very helpful,” Narasimhan says. When people start taking influenza antivirals early, she says, “it does change the course of illness and how sick they get.” Having an effective pill for SARS-CoV-2 would really help, she says.
Tamiflu has a downside: for it to be effective, it must be taken shortly after symptoms emerge. COVID-19 doesn’t progress as quickly as influenza, Narasimhan says. People with COVID-19 develop mild symptoms 3–5 days after they’ve been exposed to the virus, but their symptoms often aren’t severe enough to require hospitalization for another week after that. “If you had an oral drug that could be given to them at home and we could educate people to call when their symptoms start,” she says, it could make the disease milder. But right now, she says, “we don’t have anything really that works well.”
Remdesivir is the only antiviral approved by the US Food and Drug Administration to treat COVID-19. The drug was originally developed for hepatitis C and was tested during the 2018 Ebola virus disease outbreak in the Democratic Republic of the Congo. Gilead tested remdesivir’s activity against COVID-19 early in the pandemic and saw promising results. But remdesivir can be given only intravenously in a hospital. By the time an infection is serious enough to send someone to the hospital, the disease is usually too advanced for an antiviral to help.

Narasimhan says remdesivir has been a disappointment. “We haven’t really seen big, miraculous benefits for patients. Remdesivir really sort of hasn’t made a difference. We’ve almost shied away from using it,” she says.
“At some point with every antiviral drug, there’s a point of no return,” says Tomas Cihlar, Gilead Sciences’ vice president of virology. He explains that remdesivir suppresses the virus’s ability to copy itself by interfering with a key enzyme, the RNA-dependent RNA polymerase. But when a person’s COVID-19 infection is past a certain stage, the disease is driven by an inflammatory process, and stopping replication doesn’t have much of an effect on symptoms.
“The sooner the antiviral is administered, the better. So ideally, we would like to shift most of our impact with antivirals to outpatient settings, where the antivirals could make the most difference, primarily in preventing the hospitalizations,” Cihlar says.
To that end, Gilead has been working on an inhaled formulation of remdesivir, which is in Phase 1 clinical trials. But Cihlar acknowledges that what Gilead would really like is a version of the drug that could be taken as a pill. Remdesivir won’t work as an oral drug, though—not enough of it gets into the system to be effective when it’s swallowed.
“You have to redesign the molecule to some extent,” Cihlar says. “That’s what we’ve been doing over the last year.” Gilead scientists have been redecorating the core molecule, known as GS-441524, in an attempt to boost its oral bioavailability. They haven’t released any other details.
Other companies have also studied antivirals developed for other diseases as COVID-19 treatments. BioCryst Pharmaceuticals’ investigational antiviral galidesivir, originally developed for hepatitis C, went through Phase 1 clinical trials for COVID-19 in Brazil, but the company decided not to pursue it further.

Fujifilm’s favipiravir, which is marketed as the emergency flu treatment Avigan in Japan, has been studied in both small inpatient and outpatient trials for the past year with mixed results, says Robert Finberg, a professor of medicine at the University of Massachusetts Medical School who was involved with the trials. The drug offered little benefit to people hospitalized with COVID-19, and the results from outpatient trials, which look more promising, haven’t been published yet, Finberg says. To be effective, he says, “it probably needs to be given very early.”
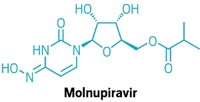
One investigational compound generating a lot of interest in the past year is molnupiravir, an oral antiviral which is being developed by Merck & Co. and Ridgeback Biotherapeutics. Last month, the companies announced they were beginning a Phase 3 clinical trial of molnupiravir for people who have COVID-19 but aren’t in the hospital. The companies scrapped plans to use the antiviral candidate in hospitalized COVID-19 patients after deciding that population is probably too sick to see any benefit from the antiviral. Merck also plans to start a trial in the second half of this year to study whether molnupiravir can prevent infections in people who have been exposed to SARS-CoV-2.

“We know from animal studies that, like influenza antivirals, antivirals against coronavirus work best when they’re used very early in infection,” says Daria Hazuda, vice president of infectious diseases and vaccine discovery at Merck. “And they work phenomenally when given to prevent transmission.”
Researchers at the Emory Institute for Drug Development started exploring molnupiravir, which they called EIDD-2801, several years ago as a treatment for viral infections including influenza and Venezuelan equine encephalitis virus. Molnupiravir interferes with a virus’s RNA-dependent RNA polymerase, leading to copies of the virus that can’t function.
The mechanism works against a broad spectrum of viruses. “Vaccines are great, but vaccines are incredibly specific,” Hazuda says. Because molnupiravir is active against many types of viruses in addition to SARS-CoV-2, it has the potential to be deployed for future outbreaks of unknown coronaviruses and for novel strains of the flu, she says. “To have an antiviral that has that sort of a broad spectrum that can be used across these kinds of pathogens is really very exciting.”
Other drugmakers are similarly focused on creating antivirals that can target multiple coronaviruses. “We want to be ready and equipped for future pandemics,” says Charlotte Allerton, Pfizer’s head of medicine design. Also, she adds, while COVID-19 vaccines appear to offer broad protection, that might not always be the case. Vaccine-resistant variants of SARS-CoV-2 could develop.
To create a broad-acting antiviral for coronaviruses, scientists at Pfizer have been working on compounds that target SARS-CoV-2’s main protease, also known as the 3CL protease. Blocking this enzyme stops the virus from breaking up long protein chains into the components it needs for reproduction. And this protease’s structure is essentially the same in all coronaviruses. That means that a small molecule that blocks 3CL in SARS-CoV-2 could also block a novel coronavirus’s 3CL, giving doctors a tool to stamp out a virus before it spreads.

Last year Pfizer announced it had an intravenous SARS-CoV-2 drug candidate—PF-07304814—in clinical trials. The compound originated in a 2003 project aimed at developing an antiviral for severe acute respiratory syndrome (SARS). The company shut down the project when that SARS outbreak was under control.
It was clear that PF-07304814 couldn’t be made into a compound that could be delivered orally, so in 2020, Pfizer scientists began a new effort to find a compound that could be. Allerton says that although the new team benefited from information gleaned in the 2003 project—the 3CL in the viruses that cause SARS and COVID-19 are essentially identical, so the researchers understood the geography of their target’s active site—the scientists were building their molecule from scratch.
Pfizer unveiled the results of that effort last month at American Chemical Society Spring 2021. The oral clinical candidate PF-07321332 was identified in July 2020 and began Phase 1 clinical trials in February. During an earnings report earlier this month, Pfizer announced plans to begin a Phase 2/3 trial of PF-07321332 later this year and to apply for an emergency use authorization for the compound before the end of 2021 if those results are positive.

Scientists at Novartis also have their sights set on an antiviral that could kill multiple coronaviruses. John Tallarico, head of chemical biology and therapeutics at Novartis Institutes for BioMedical Research, says Novartis has been working on an oral coronavirus 3CL inhibitor, although the company doesn’t expect to disclose any of its lead compounds before 2022.
Tallarico says that when Novartis started working on the project more than a year ago, it wasn’t clear that the world would still be grappling with SARS-CoV-2 now, which is why scientists there are looking for a pancoronavirus inhibitor. From March to September 2020, he says, their mantra was “this is about the next pandemic.” When it became clear that SARS-CoV-2 is going to be a global problem for years to come, their focus shifted to the current pandemic. A pill for preventing or treating the virus could help curb outbreaks, particularly in countries where supply chain hurdles, such as cold storage and distribution, make vaccinations more challenging.
Advertisement
Given the limited success of our current collection of approved antivirals, researchers hope this next batch will provide better protection for future outbreaks. But will this handful of drug candidates be enough?
“We really didn’t have much to start with during this pandemic,” NIAID’s Read says. “In terms of preventing future pandemics or responding more quickly to future pandemics, I think it would be good to have a better starting point.” Whatever scientists learn about antivirals now will give them a better foundation for addressing emerging threats.
Merck’s Hazuda says devoting more resources to developing antivirals for acute infections will help with future outbreaks. “The antiviral world, for many years, maybe decades, was focused primarily on chronic viral infections,” such as HIV infection and hepatitis C, she says. There hasn’t been much attention paid to acute infections, like SARS. “Hopefully we have learned that antivirals play a very important public health role in addition to vaccines when there are outbreaks, whether they’re seasonal outbreaks or they’re pandemic outbreaks.”
COVER STORY CONTINUES
Sign up for C&EN's must-read weekly newsletter




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter