Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
New covalent bridge found in proteins
Lysine and cysteine amino acids can be covalently linked by an oxygen atom
by Laura Howes
May 27, 2021
| A version of this story appeared in
Volume 99, Issue 20
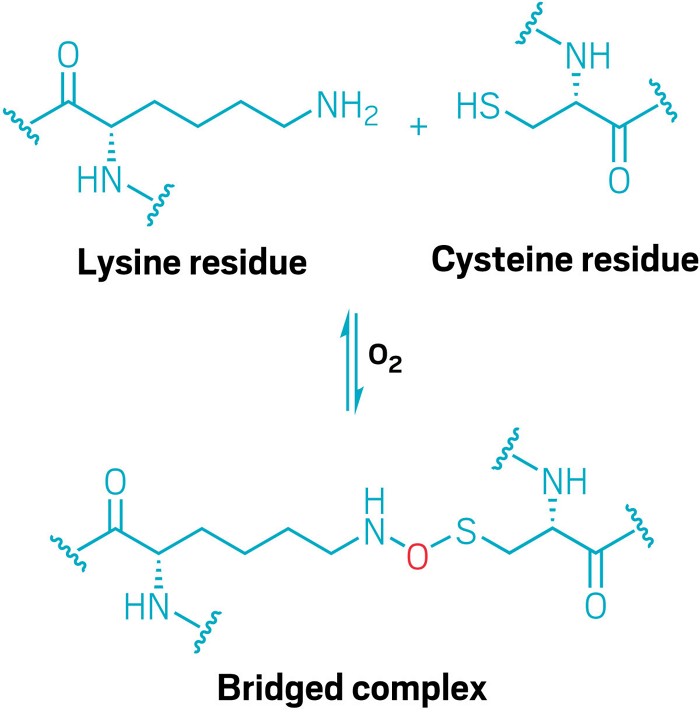
Researchers have found a new type of cross-link between amino acids in a protein (Nature 2021, DOI: 10.1038/s41586-021-03513-3). The intramolecular linkage is an N-O-S bridge formed by oxidizing the amine side group of a lysine and the thiol of a cysteine residue.
The team that made the discovery, led by Kai Tittmann at the Georg August University Göttingen, was investigating an enzyme from Neisseria gonorrhoeae called a transaldolase. When they made the protein in the lab, they found it was not active unless subjected to a reducing agent. That is usually evidence of a bridge between neighboring cysteine residues. The researchers combined data from X-ray crystallography and mutation experiments and soon realized the bridge in their enzyme is different. The covalent bond is formed by an oxygen atom that sits between the nitrogen of one amino acid and the sulfur of another and acts like a redox-activated switch for the enzyme. When oxidized, the bridge changes the protein’s shape sufficiently so that it is not catalytically active. Adding a reducing agent breaks the bridging bond, and the protein becomes active once more. Although the exact chemical mechanism for forming the N-O-S bridge is unknown at this time, the researchers looked at other protein structures in the Protein Data Bank and found evidence that their enzyme is not the only protein structure that has this previously unknown feature. How widespread the new linkage is is also unsolved so far.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter